A U.S. federal judge has ruled against compounding pharmacies, prohibiting them from producing unauthorized versions of Eli Lilly’s popular weight-loss medications, Zepbound and Mounjaro. The decision, issued in response to a lawsuit challenging the FDA’s stance on tirzepatide supply, is a significant win for the pharmaceutical giant and could impact millions of patients relying on compounded alternatives.
Legal Battle Over Compounded Tirzepatide
The ruling stems from a legal challenge brought by the Outsourcing Facility Association (OFA), a group representing compounding pharmacies. The association contested the FDA’s assertion that tirzepatide, the active ingredient in both Zepbound and Mounjaro, was no longer in short supply. Under federal law, compounding pharmacies are allowed to produce alternative versions of drugs only when a shortage exists.
The plaintiffs argued that despite the FDA’s findings, demand for tirzepatide far outstrips supply, leaving many patients unable to access Eli Lilly’s brand-name medications. They claimed that compounded versions of the drug had become a crucial option, particularly for those facing financial constraints, as many insurers do not cover weight-loss treatments.
Judge Sides with FDA and Eli Lilly
The judge ruled that the FDA’s official determination of tirzepatide’s availability was legally binding, thereby rendering the large-scale compounding of the drug unlawful. The decision effectively prevents pharmacies from producing compounded alternatives, forcing patients to seek Eli Lilly’s FDA-approved products or find other weight-loss solutions.
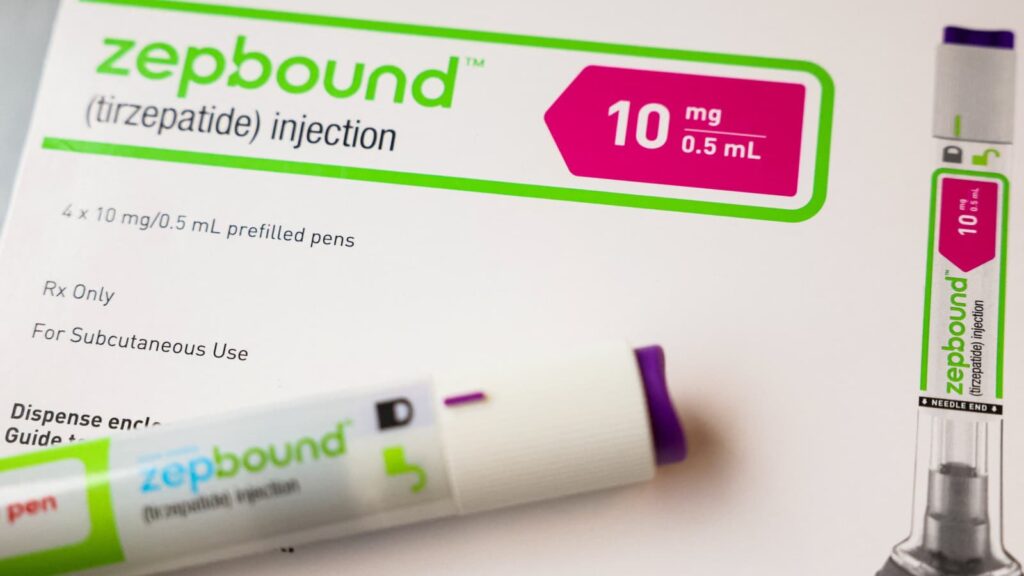
Eli Lilly applauded the ruling, stating that it reinforces patient safety by ensuring that only FDA-approved medications are distributed. “We remain committed to ensuring patients receive safe and effective treatments,” the company said in a statement.
Impact on Patients and Pharmacies
The ruling has sparked concern among patients and compounding pharmacies. Many individuals who relied on lower-cost, compounded versions of tirzepatide now face higher expenses, as compounded versions were often more affordable than the brand-name drugs. Since weight-loss medications are typically not covered by insurance, patients are frequently required to pay out-of-pocket.
For compounding pharmacies, the ruling is a major blow. Many had ramped up production to meet soaring demand, arguing that their versions provided an essential alternative amid supply shortages and high costs.
Looking Ahead
The OFA has indicated that it may pursue further legal action or appeal the ruling, arguing that patient access to weight-loss treatments should take priority over pharmaceutical exclusivity. Meanwhile, advocacy groups have called for expanded insurance coverage for obesity medications, which could help mitigate financial barriers for patients needing these treatments.
With obesity rates on the rise and demand for weight-loss solutions at an all-time high, the battle over access to tirzepatide-based medications is unlikely to end here. Whether through legal challenges, regulatory shifts, or changes in insurance policies, the conversation surrounding affordability and accessibility of weight-loss drugs will remain a pressing issue in the healthcare landscape.
Sources: Reuters, PharmaLive, Investing, MarketScreener.