A Breakthrough in Non-Opioid Pain Relief
The U.S. Food and Drug Administration (FDA) has approved Suzetrigine, the first new type of pain medication in over two decades, marking a significant advancement in pain management. The 50-milligram prescription pill, which will be sold under the brand name Journavx, offers an alternative to opioid-based treatments, providing relief without the risks of addiction and dependence.
A New Era in Pain Management
The FDA’s decision underscores the agency’s commitment to expanding pain treatment options while reducing reliance on opioids, which have fueled a national crisis of addiction and overdose deaths.
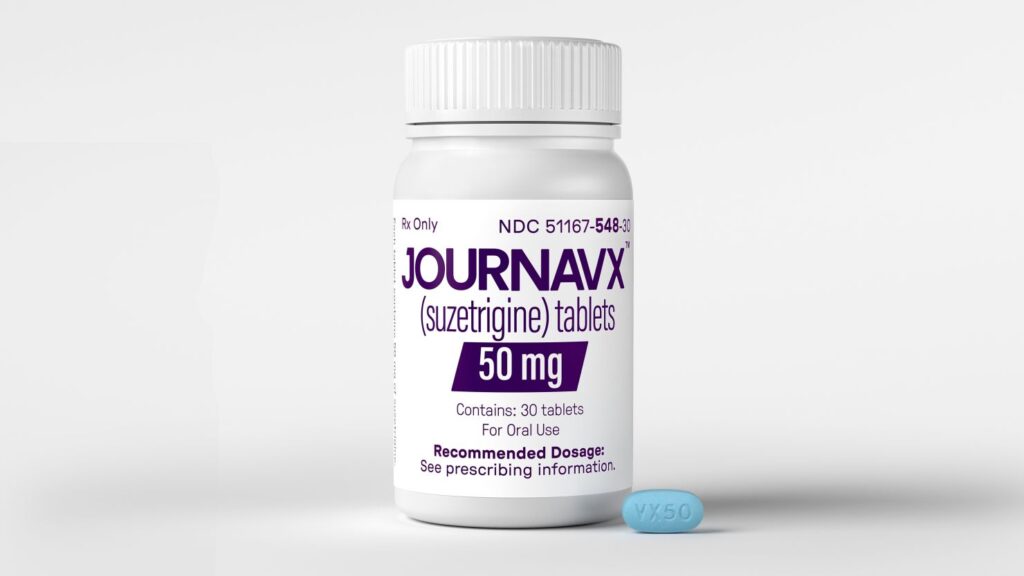
“A new non-opioid analgesic therapeutic class for acute pain offers an opportunity to mitigate certain risks associated with using an opioid for pain and provides patients with another treatment option,” said Dr. Jacqueline Corrigan-Curay, acting director of the FDA’s Center for Drug Evaluation and Research.
Each year, approximately 80 million Americans fill prescriptions for moderate to severe pain relief, with opioids making up about half of those prescriptions. Suzetrigine represents a long-awaited alternative—offering relief without the euphoric effects that contribute to opioid addiction.
How Suzetrigine Works
Pain perception involves multiple signals sent from nerve cells to the brain. Unlike opioids, which dull pain perception in the brain, Suzetrigine blocks pain signals at the source, preventing them from reaching the brain in the first place.
The drug works by blocking a specific sodium channel (Nav1.7) responsible for conducting pain signals, a mechanism first identified in a Pakistani family known for their ability to walk across hot coals without experiencing pain. Scientists discovered that a rare genetic mutation prevented their pain nerves from firing, leading to the development of Suzetrigine.
“They knew they were on something hot; they could feel the coals, but they didn’t experience pain,” explained Stuart Arbuckle, Chief Operating Officer of Vertex Pharmaceuticals, the company that developed the drug.
Clinical Trial Results: How Effective Is Suzetrigine?
Suzetrigine has undergone multiple clinical trials, including studies on patients recovering from abdominal and foot surgeries.
- Patients taking Suzetrigine reported a 50% reduction in pain, comparable to those taking Vicodin (hydrocodone/acetaminophen).
- On a 0-to-10 pain scale, patients’ pain levels dropped from 7 to about 3.5 after taking the drug.
- A study on sciatica-related pain did not show significant improvement over a placebo, raising questions about its effectiveness for chronic pain conditions.
Despite the mixed results for chronic pain, Vertex Pharmaceuticals is continuing to test Suzetrigine for long-term conditions, including diabetic neuropathy.
Implications for Pain Treatment and the Future of Non-Opioid Medications
Suzetrigine’s approval is a significant breakthrough in pain management and could pave the way for future sodium-channel-blocking drugs.
“This provides proof of concept that a sodium-channel blocker can reduce pain in humans,” said Dr. Stephen Waxman, a neuroscience expert at Yale University. “That opens the door to second-generation painkillers that could be even more effective.”
However, cost and insurance coverage could impact the drug’s accessibility. Vertex has set a wholesale price of $15.50 per pill, and insurance companies have yet to determine coverage policies.
Dr. Kimberley Mauer, an anesthesiologist at Oregon Health & Science University, emphasized the importance of having new pain treatment options but cautioned that affordability may be an issue.
“It might limit some patients from getting it, so we just have to wait and see,” she said.
Conclusion
The approval of Suzetrigine (Journavx) is a historic milestone in pain management, providing a non-opioid alternative for millions of Americans suffering from acute pain. While its full potential remains to be seen, this breakthrough offers hope for safer, more effective pain relief in the future.